The Bingel Laboratory

Prof. Dr. Ulrike Bingel and her research group focuses on the interaction between pain and cognitive processes. We have a longstanding expertise in investigating the CNS mechanisms underlying nociception, pain, and pain modulation in health and disease. In our research, we use behavioural paradigms, pharmacological modulations, as well as functional and structural brain imaging. Being particularly intrigued by the reciprocal effects of pain and cognition, we have a strong focus on translational questions such as the role of expectations and prior experiences on analgesic treatment outcomes. Our interdisciplinary research group comprises neurologists, neuroscientists, psychologists, biologists, and computer scientists and is based at the Department of Neurology at the University Medicine Essen. We are affiliated with the Erwin-L-Hahn institute for magnetic resonance imaging and the Translational Pain Research Department of the University Pain Center. Our research is funded by the Deutsche Forschungsgemeinschaft.
Recent News
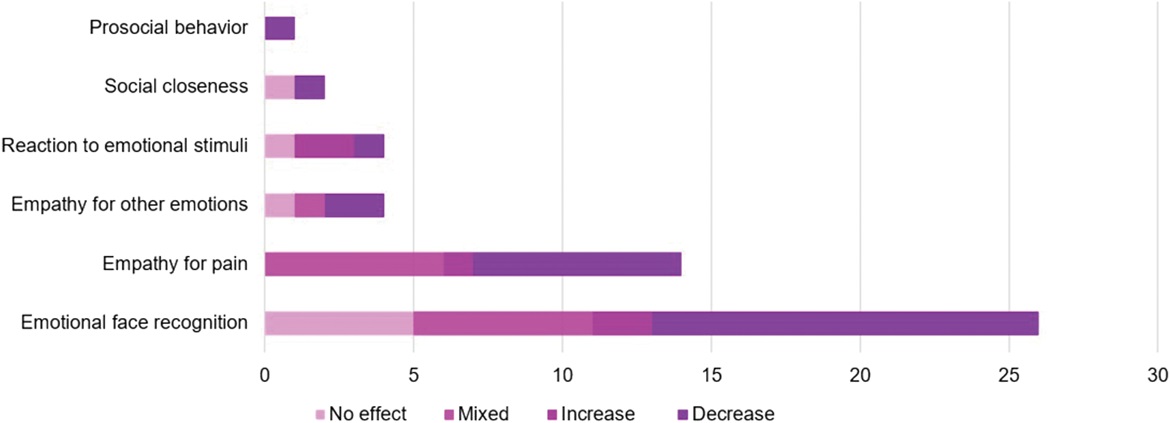
Helena Hartmann and colleagues systematically reviewed 50/2060 screened studies that looked at how changing someone’s own pain experience — through substances like opioids, acetaminophen, cannabinoids, placebos, alcohol, or hypnosis — also affects how people feel and respond to others’ emotions and pain. The authors found mixed and inconsistent effects, meaning some types of pain modulation sometimes increased or decreased social cognition abilities, with the most consistent and replicated result being that placebo analgesia reduced empathy for others’ pain. However, because the studies were very different from each other and often had small sample sizes or specific study designs, a lot more work is needed to understand how our own pain influences social feelings and behavior overall. Read the full publication in the journal Pain here.

A new (German) podcast episode from the ERCM Medizin Podcast with Ulrike Bingel talks about the power of expectations, its neurobiological mechanisms, doctor-patient communication and the importance of trust in this relationship! Watch the full episode here.

Today we have something about science communication! This new (German) book is aimed at healthcare professionals, so everyone working with or around patients: How can you use placebo effects when working with patients? How can you harness the power of expectation in patient care? How can you communicate successfully?
Order and read it here as a physical copy or ebook.
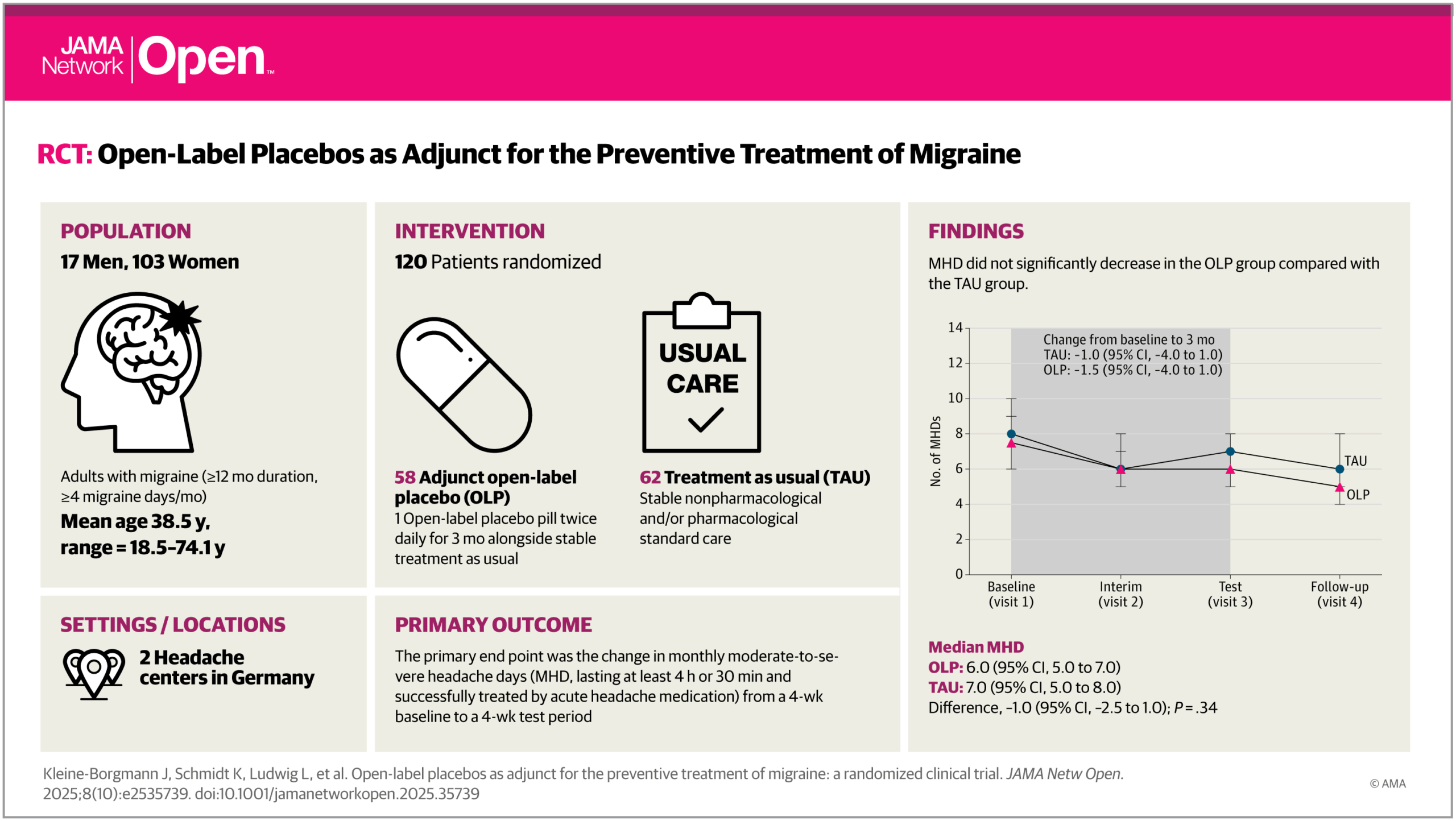
A new study from our team, published in JAMA Network Open (October 2025), shows that open-label placebos (OLPs)—placebos honestly described as such—can meaningfully improve quality of life in people with migraine when added to standard preventive treatment. While OLPs did not reduce headache frequency, patients receiving OLPs reported less pain-related disability and felt significantly better overall. These findings, led by Julian Kleine-Borgmann and Katharina Schmidt, and conducted in collaboration with the Frankfurt Headache Center within the DFG-funded CRC 289 Treatment Expectation, suggest that OLPs may represent an ethical and safe add-on option to enhance patient-centered migraine care. Read the full article in JAMA Network Open.
